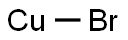Cuprous bromide: exploring its versatile applications
General Description
Cuprous bromide is a reddish-brown crystalline powder with solubility in water and certain organic solvents. It is sensitive to light, air, and moisture. Cuprous bromide exists in +1 and +2 oxidation states and undergoes hydrolysis when exposed to water. Its properties make it useful in chemistry, photochemistry, semiconductor technology, and antimicrobial research. Cuprous bromide has applications in the development of portable breath ammonia sensors for liver disease diagnosis. It is also widely used in sensor manufacturing, including optical and electrical sensors for detecting gases, bio-molecules, temperature, pressure, and electrical conductivity. Additionally, cuprous bromide acts as an effective catalyst in C-H amination reactions, enabling sustainable pharmaceutical synthesis. Overall, cuprous bromide's distinct properties contribute to its diverse applications across various fields.

Figure 1. Cuprous bromide
Properties
Cuprous bromide possesses distinct physical and chemical properties. It is typically found as a reddish-brown crystalline powder or solid and exhibits solubility in water, alcohols, and certain organic solvents. However, it is sensitive to light, air, and moisture, necessitating proper storage and handling. Furthermore, cuprous bromide primarily exists in the +1 oxidation state (cuprous ion) but can also form compounds in the +2 oxidation state (cupric ion). When exposed to water, it undergoes hydrolysis, resulting in the formation of copper hydroxide and hydrogen bromide gas. These properties enable its application in various fields, including chemistry, photochemistry, semiconductor technology, and antimicrobial research. Careful consideration of these characteristics is crucial for effectively utilizing cuprous bromide in different scientific and industrial contexts. 1
Applications
Real-time breath ammonia measurement
The application of cuprous bromide in developing a portable sensor device for real-time measurement of breath ammonia levels. A study aimed to evaluate the practical usability of the cuprous bromide sensor device and its correlation with blood ammonia in patients suffering from chronic liver disease (CLD). This was a feasibility and pilot clinical study involving 21 CLD patients and 18 healthy volunteers. The researchers used a p-type semiconductor cuprous bromide thin film to directly and rapidly measure breath ammonia levels with high sensitivity and selectivity. In conclusion, the newly developed, easy-to-use, and portable cuprous bromide sensor device demonstrated its capability to non-invasively measure breath ammonia in real time. The measured breath ammonia showed a correlation with blood ammonia levels and the presence of liver cirrhosis, suggesting its potential as a surrogate biomarker for blood ammonia. 2
Sensor manufacturing
Cuprous bromide is a compound widely used in sensor manufacturing. In optical sensors, it is employed in coatings that have high absorption and scattering capabilities. These coatings enable the detection and measurement of various analytes, including gases and bio-molecules. Cuprous bromide is particularly useful in the production of gas sensors used to detect toxic gases like carbon monoxide and ammonia. In electrical sensors, cuprous bromide serves as a conductive material with superior electrical conductivity and thermal stability. This makes it ideal for manufacturing sensors that detect changes in temperature, pressure, and electrical conductivity. For example, cuprous bromide is applied in pressure sensors used in industrial processes to monitor pressure levels and ensure efficiency. Overall, cuprous bromide plays a vital role in sensor development across various fields. Its presence improves the capabilities and accuracy of sensors in detecting and measuring a wide range of physical and chemical phenomena, empowering advancements in research, industry, and environmental monitoring. 3
Catalyst
Cuprous bromide is an effective catalyst in the direct C-H amination of arenes. It utilizes NHPI as the amidyl radical precursor and works under ambient conditions. The reaction offers numerous benefits, including direct C-H bond functionalization and high product yields. Cuprous bromide facilitates the generation of amidyl radicals from NHPI through electron transfer, while triethyl phosphite acts as a hydrogen atom transfer agent. The cuprous bromide/TEP system promotes the recombination of carbon-centered radicals with amidyl radicals, resulting in C-H amination. This methodology provides a sustainable approach for synthesizing pharmaceuticals and valuable organic compounds, showcasing cuprous bromide's importance in catalysis. 4
Reference
1. PubChem. COMPOUND SUMMARY: Copper(1) bromide. National Library of Medicine, 2005, CID: 24593.
2. Ishida J, Oikawa T, Nakagawa C, Takano K, Fujioka K, Kikuchi Y, Tsuboi O, Ueda K, Nakano M, Saeki C, Torisu Y, Ikeda Y, Saruta M, Tsubota A. Real-time breath ammonia measurement using a novel cuprous bromide sensor device in patients with chronic liver disease: a feasibility and pilot study. J Breath Res, 2021, 15(2):026010.
3. Ran Z, Cao S, Peng Q, Liu X, Zhou J. Deep-Red Luminescent Cuprous-Lead Bromide as a Dual-Responsive Sensor for Fe3+ and Cr2O72. Inorg Chem, 2022, 61(15):5957-5964.
4. Zhang D, Lv B, Gao P, Jia X, Yuan Y. Direct C-H amination reactions of arenes with N-hydroxyphthalimides catalyzed by cuprous bromide. Beilstein J Org Chem, 2022, 18:647-652.
You may like
Related articles And Qustion
Lastest Price from Cuprous bromide manufacturers

US $100.00-80.00/KG2025-07-08
- CAS:
- 7787-70-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000000

US $10.00/KG2025-04-21
- CAS:
- 7787-70-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt